Abstract

Background: Myeloablative HLA-matched sibling donor hematopoietic cell transplantation (HCT) is highly curative for children and young adults with sickle cell disease (SCD). The most common regimen used worldwide has been Busulfan, Cyclophosphamide and anti-thymocyte globulin (ATG). The optimal type of ATG for this approach remains unclear.
Objective: To compare HCT outcomes of horse ATG (hATG) versus rabbit ATG (rATG) in patients with SCD undergoing HCT.
Methods: Multicenter retrospective data from patients with SCD undergoing HCT were abstracted from the Sickle Transplant Alliance for Research (STAR) database. Patients undergoing myeloablative and reduced intensity conditioning HCT with matched related donors where the conditioning regimen included ATG were included in the analysis. Baseline characteristics were compared between the groups using chi-square and Wilcoxon Rank-Sum tests. Kaplan-Meier curves were used to assess time-to-event outcomes, such as overall and event free survival (EFS), and compared with log-rank tests. Death, disease recurrence (acute sickle cell symptoms/HbS >50%) and graft failure (whole blood or myeloid chimerism < 5%) were considered events for EFS. Full donor chimerism was defined as ≥95% donor. P-values less than 0.05 were considered statistically significant.
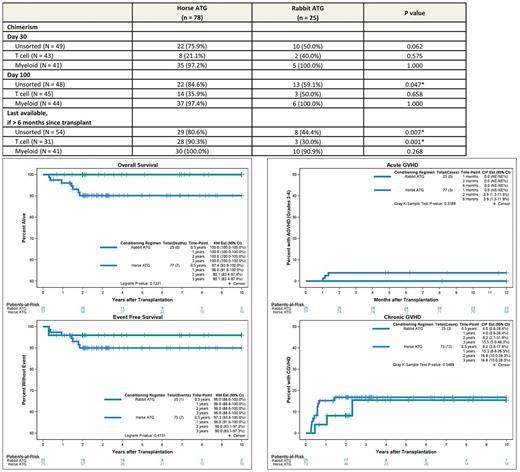
Results: 103 patients (hATG = 78, rATG = 25) from 7 U.S. centers and one Canadian site were available in the database for analysis. Median age at HCT (8.1 years vs 7.6 years, p = 0.866) and gender distribution (55.1% vs 56.0% male, p = 0.939) was similar between the cohorts. Sickle cell genotype was 97.4% vs. 84% HbSS, 1.3% vs. 12.0% HbSBetazero, 1.3% vs. 0% HbSC, and 0% vs.4% other. Pre-transplant disease acute symptoms were similar between the groups. Myeloablative conditioning was used in 92.3% and 96.0% in the hATG group versus the rATG group (p=1) and the most common conditioning regimen was Busulfan and Cyclophosphamide +/- Fludarabine in both groups (93.2%). Donor age and source of graft were similar between the two groups. Median follow-up for hATG and rATG groups were 3.0 and 3.6 years respectively. The year of transplant for the two groups ranged from 1993 to 2015 (hATG) and 2003 to 2014 (rATG). Three-year OS for the entire group was 92.6% (95% CI: 87.4-98.0), with 90.1% (95% CI: 83.4-97.4) in the hATG group and 100% (95% CI: 100-100) in the rATG group (p = 0.122) (Figure 1). Three-year EFS was 90% vs. 96% (p=0.415) in hATG vs. rATG group with 2-year graft failure incidence of 0% vs. 4% (p =0.085) respectively. Limiting the analysis to transplants performed after 2000 did not materially alter the analyses for OS and EFS. Causes of death were GVHD related (n=5) and infection/sepsis (n=3) in the hATG group versus no deaths in rATG group. Six month incidence of acute grade III-IV GVHD and 3 year incidence of chronic GVHD for hATG vs. rATG groups was 3.9% vs. 0% (p=0.319) and 16.8% vs. 15.5% (p=0.549) respectively. Prevalence of full donor myeloid chimerism was similar between the two groups at Day +30, Day +100 and >6months. Prevalence of full donor whole blood chimerism was significantly lower at day+100 and 1 year (p < 0.05) in the rATG group. Full donor T cell chimerism was similar at Day 30 and Day 100, but significantly less prevalent at >6 months in the rATG group (p = 0.001) (Table 1).
Discussion/Conclusion: In our cohort mortality was low overall and the hATG and rATG groups had similar OS, EFS, and similar incidence of acute grade III-IV and chronic GVHD. However the prevalence of full donor T cell chimerism at greater than 6 months after HCT was significantly lower in the rATG group.
Table 1. Incidence of full donor chimerism (≥ 95%) by lymphocyte depleting antibody, %
Fig 1. Overall and Event Free survival of hATG vs. rATG based conditioning regimens in HCT for SCD
Fig 2. Incidence of acute grade III-IV and chronic GVHD in hATG vs. rATG based conditioning regimens in HCT for SCD
Guilcher: Jazz pharmaceuticals: Consultancy; Canada Market Research: Consultancy; Patient Access Solutions: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal